Wine Industry
- Project Manager for fully-automated, high capacity bottling line for WBM Top-30 winery. Incorporated project tenants of flexibility, quality, efficiency, synchronization, and automation to identify innovative, leading edge equipment capable of running cased and bulk glass.
 Project Engineer for Scope Definition phase of a greenfield, multi-use winery facility comprised of bottling, warehousing, and barrel handling operations in the Napa Valley. Developed detailed scope definition documents outlining all equipment, facility, and utility requirements for this state-of-the-art installation. Specified design and performance requirements for each piece of equipment on the 12,000 case per day contract bottling line. Identified and specified tenant improvements for the entire 150,000 s.f. facility. Acted as Owner’s Representative to developer/contractor.
Project Engineer for Scope Definition phase of a greenfield, multi-use winery facility comprised of bottling, warehousing, and barrel handling operations in the Napa Valley. Developed detailed scope definition documents outlining all equipment, facility, and utility requirements for this state-of-the-art installation. Specified design and performance requirements for each piece of equipment on the 12,000 case per day contract bottling line. Identified and specified tenant improvements for the entire 150,000 s.f. facility. Acted as Owner’s Representative to developer/contractor.- Co-leader of project team that executed the complete redesign of a full line of wine packaging for a large Napa Valley winery. Responsible for equipment specification, procurement, and installation. This package earned an AmeriStar Package Award.
- Project Manager and Engineer for the design and installation of a new bottling line created by combining equipment and conveyor components from three existing lines.
- Production Engineer responsible for coordination and execution of multiple projects ranging in scope from package design and testing through packaging and process equipment specification, acquisition, and installation for a six-million plus case winery.
- Project Engineer for winery cellar and crush pad expansion. Cooperage project added 4 million gallons of refrigerated cooperage on two sites including catwalks, wine transfer lines, and associated structures. Quintupled press capacity with the largest multiple grape press installation in the industry to that date; eight 50,000 liter bladder presses fed by a progressing cavity pump and extensive pomace discharge system.
- Project Manager for installation phase of fast-track project to upgrade spirits processing facility in support of new products. Scope included adding stainless cooperage with associated pumps and instrumentation, new product transfer lines, modifications to existing piping, new RO filter, and modifications to hot water boiler system.
- As Project Engineer, developed User Requirements Document for new bottling line equipment from de-casing through load building. Document clearly defined equipment requirements for a line capable of bottling full range of 750 ml package configurations including CIP-capable filler, cold glue and pressure sensitive labeling, cork and ROPP closures, label and case coding, and all conveying systems.
Life Sciences
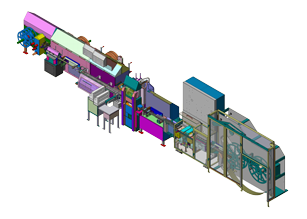 Owner’s Representative for two highly specialized, custom, form/fill/seal machines to produce inhalable insulin strips for use in innovative medical device. Increased organization and communication internally between client team members and between client and custom equipment vendor. Responsible for facilitating decision-making, balancing priorities, and maintaining time-critical project schedule.
Owner’s Representative for two highly specialized, custom, form/fill/seal machines to produce inhalable insulin strips for use in innovative medical device. Increased organization and communication internally between client team members and between client and custom equipment vendor. Responsible for facilitating decision-making, balancing priorities, and maintaining time-critical project schedule.- Project Manager and Engineer for project to implement laser coding and Optical Character Verification (OCV) of lot code and expiration date information on pouched pharmaceutical products. Performed and documented vendor comparison of laser coding equipment manufacturers. Responsible for developing User Requirements and Scope Definition in conjunction with the client’s user groups, and for specifying all equipment, including a new pouch stock payout station to allow for off-line integration and validation.
- Project Manager for three semi-automated work cells for discreet assembly functions on assay cartridges for a novel rapid point-of-care (POC) test for identification and quantification of absolute counts of CD$ T-Lymphocytes in whole blood to guide HIV/AIDS treatment in resource limited countries.
- Project Engineer for development and validation of a custom-designed system for packaging and shipping frozen intermediate pharmaceutical product cross-country. The innovative design protects and maintains the temperature of the fragile contents well below -30°C for over 96 hours and earned a 2000 AmeriStar Package Award and AmeriStar’s Specialty Award for Best Protective Package.
- Project Engineer for the acquisition, validation, and start-up of a custom automatic inspection and reject system used for lyopholized paranteral drugs in a Class 100 clean room. As a consultant, Andy was recognized as a team member for the client’s own President’s Achievement Award for continuous improvement.
- Developed ‘Best Practices’ document for pharmaceutical manufacturing company to facilitate creation of training video to enhance efficient operation of subsystems on custom packaging machine.
- Performed an efficiency study on a validated pharmaceutical packaging line performing vial labeling, automatic inspection, and secondary and tertiary packaging for lyopholized parenteral products. The scope of work included extended on-site observation and data acquisition periods supported by employee interviews. The results were analyzed and key issues identified. Recommendations were prioritized and categorized into mmediate and long-term process improvements including implementation of
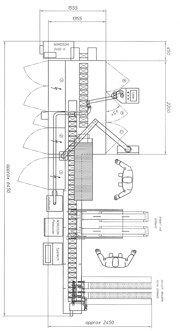 Disturbance Frequency Analysis program to monitor results and effect continuous process improvements moving forward. Client implemented several recommendations outlined in the report.
Disturbance Frequency Analysis program to monitor results and effect continuous process improvements moving forward. Client implemented several recommendations outlined in the report. - Project Engineers for two custom cartoning machines for pharmaceutical packaging operations in two separate facilities.
- Project Engineers responsible for preparation of specifications for purchase of a wide variety of packaging machinery for pharmaceutical manufacturers including pouching, tray-loading, cartoning, automatic inspection, and f/f/s equipment.
- Project Engineer for the specification and acquisition of custom filling and packaging equipment for innovative new ‘smart patch’ drug delivery product for smoking cessation.
- Packaging SME for acquisition of unique packaging line combining liquid and solid dose filling with shared labeling, cartoning, bundling, case packing, and palletizing equipment.
- Project Manager for acquisition, installation, start-up, and testing of two high-capacity (filling through case packing) oral solid dosage packaging lines, equipped for track-and-trace.
- Packaging SME and Project Manager for the acquisition of two complete packaging lines for a green field project. Lines included one for solid dose tablets and one for vials of lyophilized products; both equipped for track-and-trace.
- Project Engineer for purchase, installation, and start-up of custom cartoning machine for packaging of pouched, transdermal products. Cartoner included 100% verification of product bar codes and printing and verification of lot and expiration dates on the cartons. PUT FURTHER DOWN ON LIST.
- Owner’s Representative on project to purchase custom, automated machine for assembling inhalable drug delivery device sub-assemblies. The $2 million system incorporated multiple automated vision inspection and test stations and three six-axis robots.
- Project Engineer for project to upgrade secondary and tertiary packaging equipment to accommodate innovative new closure / delivery system on parenteral drug vials. Included upgrades to automatic labeling, inspection, coding, and cartoning systems.
- Project Engineer on project to automate the loading and unloading of parenteral drug vials into and out of bulk shippers for transfer from filling / freeze-drying site to packaging facility. Collaborated with client’s stakeholders to develop preliminary user requirements, solicited schedule and budgetary pricing, and presented options for capital justification package in client’s format.
- Performed ‘Automatability Assessment’ for existing, manual process to assemble production quantities of disposable components for a non-opioid, pain treatment device. Consideration given to suitability of existing technologies and risk associated with rapid scale-up.
Manufacturing
- Maintenance Supervisor responsible for all maintenance and capital projects for all automatic inspection, carton erecting, case packing, load building, and product handling equipment for a nine-machine glass container manufacturing facility.

